Unique selling points
- No heating
- Low cost
- High efficiency
- Possible mineralization
- Less harmful by-products
- Universal method
Advanced Oxidation Processes (AOPs) constitute a suite of chemical treatment techniques utilized for purifying wastewater. These methods involve the in-situ generation of highly reactive oxygen species (ROS), primarily hydroxyl radicals (•OH), which are potent oxidants capable of breaking down and mineralizing nearly any chemical compound into environmentally benign CO2 and inorganic ions (Malato et al., 2009, Wols et al., 2012). Key AOPs encompass practices such as ozonation, photocatalysis (employing materials like titanium dioxide), and the application of Fenton's reagent (involving hydrogen peroxide with iron catalysts), each tailored to address diverse pollutants and environmental conditions.
AOPs are typically employed when conventional treatment methods prove inadequate due to the complexity or persistence of contaminants, such as:
- Compounds with low biodegradability that are not effectively removed by biological treatments, or toxic substances.
- Toxic compounds that inhibit microorganisms in biological treatments.
- Predatory organisms that consume microorganisms used in biological treatments.
Description of the technology
In certain AOPs, such as TiO2 photocatalysis and the photo-Fenton process, the generation of ROS can be augmented by light. Some research has focused on developing AOPs driven by sunlight (Byrne et al., 2015, Tsydenova et al., 2015), leveraging renewable and cost-free solar energy to potentially reduce treatment expenses and enhance environmental sustainability (Munoz et al., 2006). Solar-enhanced methods are particularly advantageous for regions abundant in sunlight. Furthermore, AOPs' capability to simultaneously eliminate pathogens and chemical pollutants could enhance the economic efficiency of water and wastewater treatment by integrating disinfection and pollutant removal, traditionally treated as separate processes, into a single treatment step.
Photocatalytic activity is the property of a solid material induced by the irradiation of photons with energy equal to or greater than the bandgap energy. This causes the valence band (VB) electrons to be excited to the conduction band (CB) and leave holes in the former. In this way, electron-hole pairs (e⁻-h⁺), called excitons, are generated, which can subsequently be utilized to carry out redox reactions.
Figure 2 represents a schematic of the photocatalytic process on the surface of TiO₂ for the oxidation of contaminant compounds. The process occurs as follows:
- Photon absorption: TiO₂ absorbs photons with energy equal to or greater than its bandgap energy (approximately 3.2 eV for anatase).
- Generation of electron-hole pairs: the photon energy excites electrons from the valence band (VB) to the conduction band (CB), leaving holes in the valence band.
- Charge separation: electrons (e⁻) in the conduction band and holes (h⁺) in the valence band separate and migrate to the surface of the TiO₂.
- Surface redox reactions:
- Oxidation of contaminants: holes (h⁺) on the surface of TiO₂ can directly oxidize contaminant compounds, converting them into less harmful products.
- Formation of hydroxyl radicals: holes (h⁺) can react with water molecules or adsorbed hydroxyl groups to form hydroxyl radicals (•OH), which are powerful oxidizing agents capable of degrading organic compounds.
- Oxygen reduction: electrons (e⁻) in the conduction band can reduce adsorbed molecular oxygen (O₂) on the surface to reactive species like superoxide (O₂•⁻).
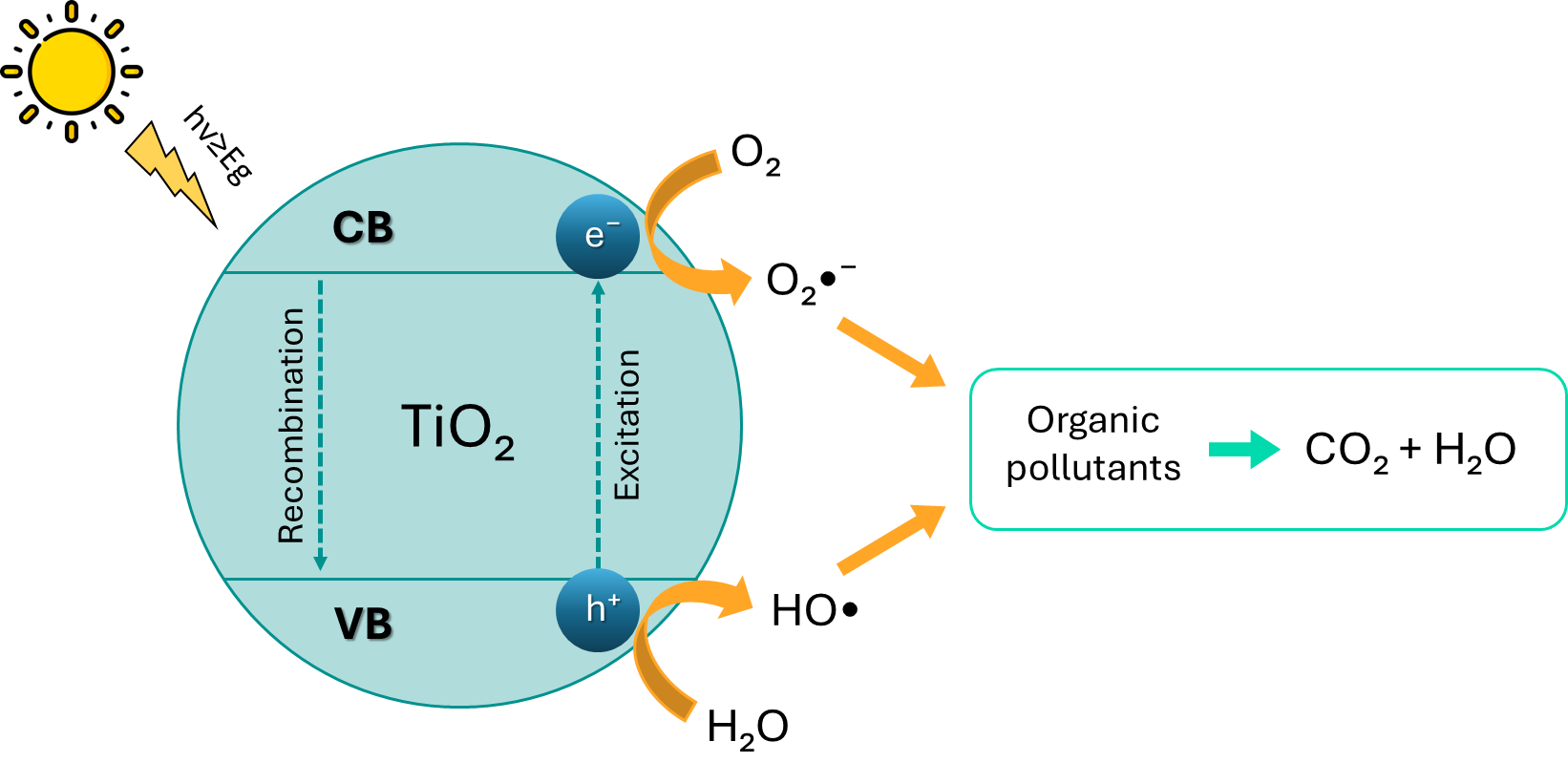
Figure 2. Photocatalytic process on the surface of TiO₂ for the oxidation of contaminant compounds.
In the framework of the ULTIMATE project, in the Case Study 4 has developed two AOP reactors, the Compound Parabolic Collector (CPC) photocatalytic reactor that is mainly used as a solar photocatalysis reactor using titanium dioxide and an annular reactor for artificial UV light irradiation with hydrogen peroxide (H2O2).
The CPC is a type of trough concentrator technology that focuses solar energy onto a receiver tube. The reflector geometry is constructed by combining two symmetrical parabolic segments with different focal lengths. CPCs can achieve a concentrating ratio close to one, capturing both direct and diffuse UV sunlight. This design ensures nearly all UV radiation entering the CPC is utilized in the reactor, with light reflected around the reactor tube for uniform illumination (Malato et al., 2002). The simplicity, cost-effectiveness, and low capital investment of CPCs, along with efficient water distribution, contribute to their superior performance in solar photochemical and photocatalytic applications (Malato et al., 2002).
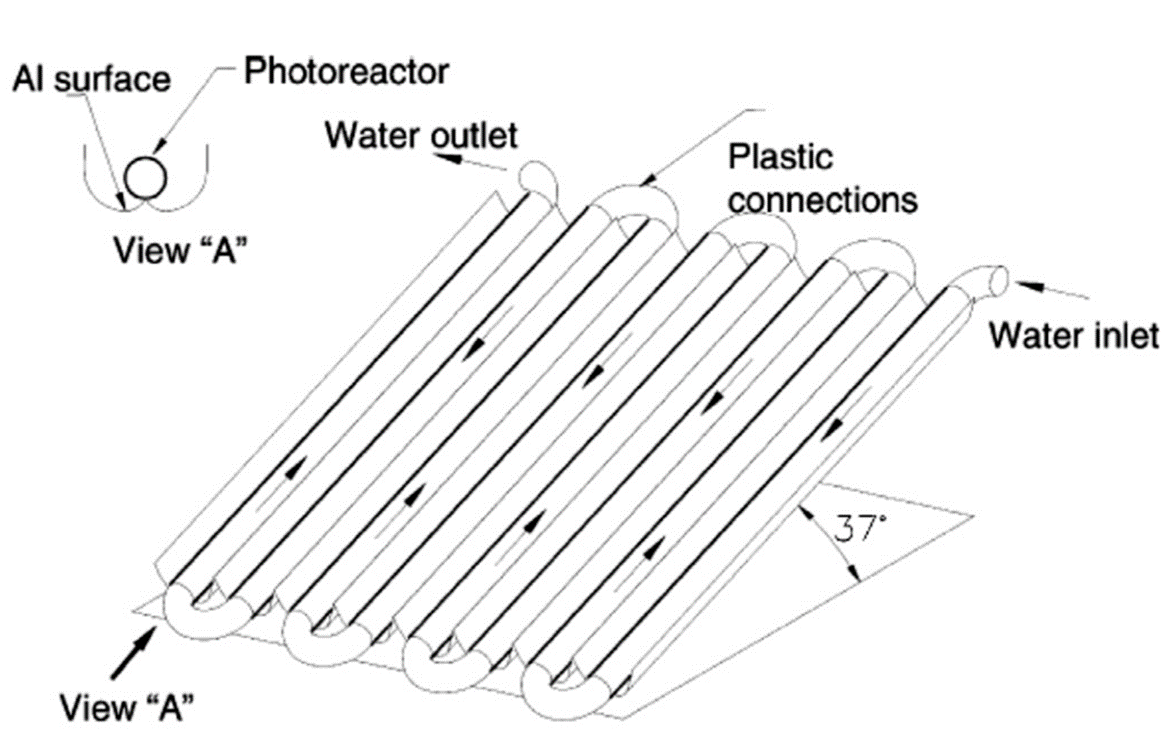
Figure 3. Schematic drawing of a CPC (Malato et al., 2002).
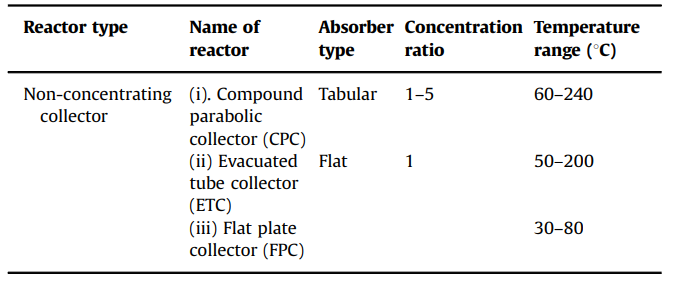
Table 1. Different types of non-concentrating collectors (Tanveer et al., 2013)
Flow scheme of the technology
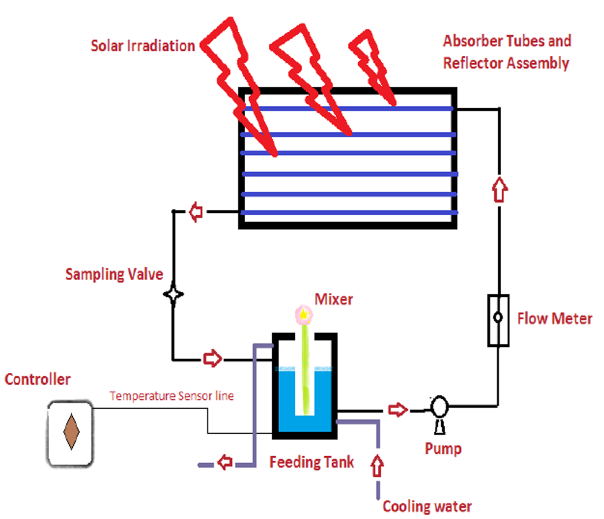
Figure 4. CPC scheme (Tanveer et at., 2013)

Figure 5. Picture of the CPC Solar Reactor

Figure 6. Picture of the Annular AOP Reactor
Synergetic effects and motivation for the implementation of the technology
AOPs combined with solar energy provide several advantages:
- Enhanced efficiency: utilizing sunlight for the processes can significantly reduce energy costs.
- Environmental sustainability: leveraging renewable solar energy helps in reducing the carbon footprint of wastewater treatment.
- Economic efficiency: combining disinfection and pollutant removal into a single step streamlines operations and reduces overall treatment costs.
- Broad applicability: AOPs are effective against a wide range of contaminants, including those resistant to conventional treatments.
Key performance indicators
Implementing Advanced Oxidation Processes (AOPs) technology for wastewater treatment involves several key requirements to ensure optimal performance and efficiency. These requirements can be broadly categorized into infrastructure, operational, and environmental considerations.
Infrastructure requirements
- Reactor design and configuration:
- Photocatalytic reactors: requires specific designs such as Compound Parabolic Collectors (CPC) or annular reactors to optimize the capture and utilization of light (solar or artificial UV).
- Material selection: high-quality materials like titanium dioxide (TiO₂) for photocatalysis must be used.
- Light sources:
- Solar reactors: sufficient sunlight exposure is essential for solar-driven AOPs. This may require geographic considerations for optimal placement.
- Water and wastewater management:
- Piping and distribution systems: efficient systems to ensure proper flow and mixing of water and reagents within the reactors.
- Pre-treatment facilities: systems to remove large particles and reduce turbidity to enhance the efficiency of AOPs.
Operational requirements
- Chemical reagents:
- Consistent supply of H₂O₂.
- Monitoring and Control Systems:
- Sensors and instruments: devices to monitor pH, temperature, dissolved oxygen, total organic carbon and other critical parameters.
- Automation and control: systems for the automated addition of reagents and control of operational parameters to maintain optimal conditions.
- Maintenance and upkeep:
- Regular maintenance: routine checks and maintenance of reactors, light sources, and other equipment to ensure consistent performance.
- Cleaning protocols: methods for cleaning reactor surfaces, especially for photocatalytic reactors, to prevent fouling and maintain efficiency.
Environmental and safety requirements
- Environmental conditions:
- Climate considerations: for solar AOPs, adequate sunlight availability is crucial, which may require geographical and seasonal planning.
- Safety measures:
- Chemical handling: safe storage and handling protocols for reactive chemicals like H₂O₂.
- UV safety: protective measures to shield operators from harmful UV exposure.
- Regulatory compliance:
- Environmental regulations: compliance with local and international regulations regarding wastewater discharge and treatment processes.
- Health and safety standards: adherence to occupational health and safety standards to protect workers.
Resource and economic considerations
- Cost-Effectiveness:
- Capital investment: initial costs for setting up reactors and associated infrastructure.
- Operational costs: ongoing costs for reagents and maintenance.
- Scalability and flexibility:
- Scalable solutions: ability to scale the technology to meet varying wastewater treatment demands.
- Adaptability: flexibility to adapt the AOP system to different types of contaminants and wastewater characteristics.
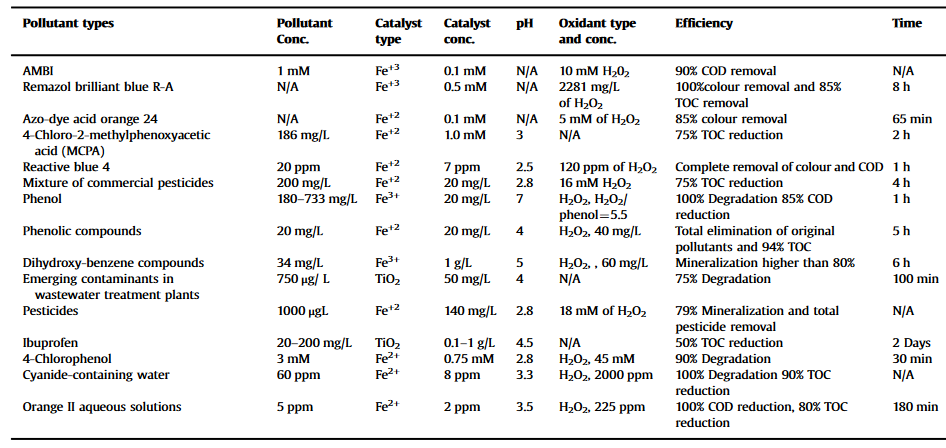
Table 2. CPCs operation conditions in wastewater treatment (Tanveer et al., 2013)