Unique selling points
- Selective ion separation
- High product recovery
- No phase change leading to less energy input
- Limited chemical requirement
Description of the technology
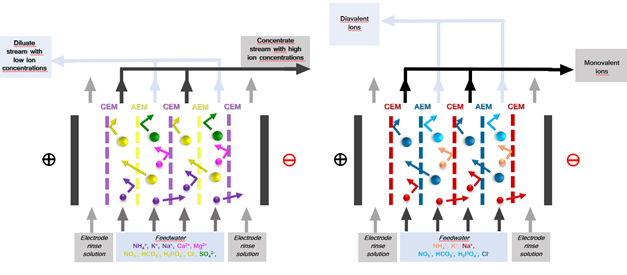
Electrodialysis (ED) is an electrochemical separation process that (selectively) transports ions through electrostatically charged ion-exchange membranes (IEMs) driven by an electrical potential difference (applied electric field). ED is a well-established technology with applications in treating brackish water, industrial wastewater, municipal wastewater, table salt production, heavy metals removal, and acid and bases production (Sajjad Al-Amshawee, 2020). The increase in popularity and application of ED has been accelerated by the development of novel IEMs with improved electrochemical and physio-chemical properties. ED is a promising technology for the application of treating and potentially reusing greenhouse horticulture wastewater, as it has the ability to selectively remove ions such as sodium (which is harmful for crop growth) and retain nutrients (which can be used as fertilizers). Recent research for this application has been dedicated to lab-scale ED configurations (Yvana D. Ahdab, 2021). Within ULTIMATE, lab-scale to pilot-scale investigations are developed to validate the feasibility of ED application in the greenhouse horticulture industry for water and nutrient reuse (CS Nieuw Prinsenland).
An ED stack consists of alternating anion exchange membrane (AEM) and cation exchange membranes (CEM) arranged in series between an anode and a cathode (Huffmann, 1972) (Fig 1). An electric potential difference is set between the anode and cathoda when an ionic solution flows through the cells, resulting in the positively charged cations being attracted by the cathode and negatively charged anions by the anode. The positively charged cations pass through the negatively charged CEM but are retained by the AEM. Likewise, the negatively charged anions pass through the AEM and are retained by the CEM. This results in a concentration of ions in alternating compartments (concentrate) and simultaneous depletion of ions in the others (diluate). (Strathmann, 2010). Selectivity in ED can be further enhanced by employing monovalent selective membranes (MVMs) – anionic (MVAs) and Cationic (MVCs) – to separate monovalent and multivalent ions. Furthermore, Selectrodialysis (SED) can be configured with 3 compartments (1 MVM and 2 IEMs fractionalizing ions into 3 streams) (Yang Zhang, 2012).
Flow scheme of the technology
The ED stack consists of alternating anion exchange membrane (AEM) and cation exchange membranes (CEM) arranged in series between an anode and a cathode (Huffmann, 1972) (Fig 1). Spacer gaskets are used inside the ED stack to create concentrate and diluate compartments by separating the IEMs. An electrolyte solution – electrode rinse solution (ERS) – is circulated in the electrode compartments. Feedwater is pumped through cells, and an electrical potential is established. Depending on the type of membrane, configuration, and operating conditions used (IEMs, MVMs etc.), f,the eed stream is split into a diluate and concentrate stream and/or recirculation and product recovery streams. Additionally, selective ion separation can be achieved by employing specific IEMs to separate unwanted ions or concentrate value-added products. For example, cations like Na+, K+, and NH4+ will pass through the CEM towards the cathode compartment, and anions like Cl-, SO4- and PO4- will pass the AEM towards the anode, providing electrical balance to in the water matrix (C. Femina Carolin, 2017).
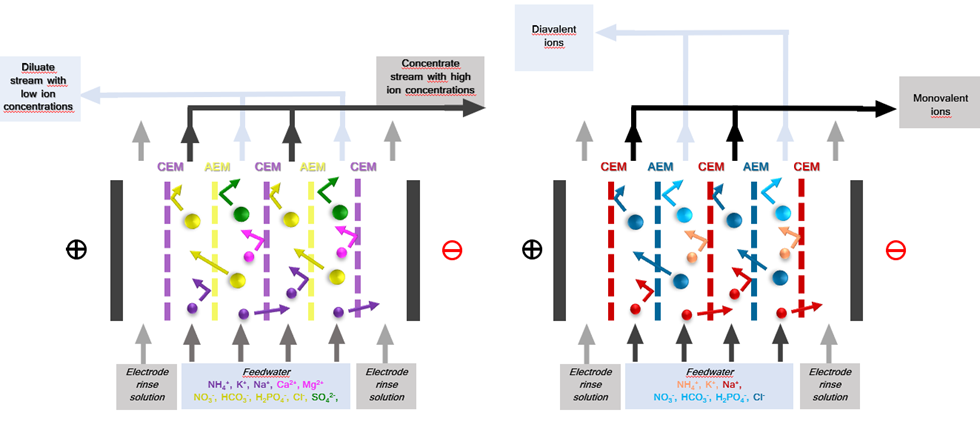
Fig. 1 Electrodialysis (ED) set-up with ion-exchange membranes and monovalent selective ion exchange membranes
Synergetic effects and motivation for the implementation of the technology
- Selective ion separation for high product recovery
ED’s main industrial application is the desalination of brackish water for producing potable water (Tanaka, 2007) (Strathmann H. , 2010), followed by salt production by seawater concentration (Luigi Gurreri, 2020). Furthermore, its application has been studied in many fields and for the recovery of valuable products, including nutrients from (waste)water. In contrast to other separation technologies that separate large water volumes from solutes, electricity-driven processes remove ions, enhancing ion-selective recovery (William A. Tarpeh, 2021). Additionally, researchers have also examined separating organic nitrogen and inorganic nitrogen (Kangmin Chon, 2013), and ionic nutrients and organic substances (Yang Zhang B. V., 2009).
- No phase change and limited chemical requirement
Trading chemical additions for electricity, especially from renewable sources, can offset substantial impacts from water treatment processes. Selective separaton by electrochemical systems are quite suitable as they utilize electricity as their main input, improve process control, and have lower transport-based energy and chemical inputs (Ll. Corominas, 2013).
Technology requirements and operating conditions
For ED and processes founded on ED, the treatment potential can range from below 100 m3/day to 20,000 m3/day (Strathmann H. , 2010). The two main ED geometric patterns include sheet flow and the tortuous path, and it can operate in batch or continuous mode (Luigi Gurreri, 2020). Table 1 Provides the range of typical ED operating parameters.
Tab. 1 Typical ranges for operating parameters
|
Parameter |
Units |
Range |
Reference |
|
Cell pairs |
no. |
<10 (bench) – 100s (pilot/full) |
|
|
Active area per membrane |
m2 |
0.01 – 0.06 (upto 1) |
(Gurreri L, 2020), (M. Demircioglu, 2003) |
|
Spacer thickness |
mm |
0.3 - 2 |
|
|
Fluid velocity |
cm/s |
1-10 (upto 50) |
|
|
Applied voltage |
V |
7-30 (lab scale) |
|
|
Current density |
mA/cm2 |
5 - 60 |
|
|
Running time |
h |
24, 30, 55, 72 |
Key performance indicators
Depending on the types of wastewater source and ED process, water recovery and product concentration can vary, as shown in Tab 2.
Tab. 2 Product concentration and water recovery for different types of wastewaters in ED processes
|
Wastewater |
Method |
Product concentration |
References |
|
Synthetic municipal wastewater |
Selective ED |
1.6 mmol L−1 NO3−, 0.68 mmol L−1 PO4−3-P |
|
|
Anaerobic digesting excess sludge |
Hybrid ED + struvite |
80 mmol L−1 PO4−3-P, 600 mmol L−1 NH4+1-N |
|
|
Secondary effluent |
Selective electrodialysis |
22 mg L−1 N, 40 mg L−1 P |
|
|
|
|
Recovery |
|
|
Industrial wastewater |
Electrodialysis |
85% freshwater recovery |
For greenhouse wastewater, (Yvana D. Ahdab, 2021) observed in their laboratory experiments that selective CEMs could remove from 1.3 – 3.2 sodium ions for every calcium ion and 2-7.6 sodium ions for every magnesium ion. Selective AEMs could remove 2.3-6.5 nitrate ions for every sulfate ion and 1.1 – 6.5 nitrate ions for every phosphate ion.
Case Studies applying the technology
Publications
- Naves Arnaldos, A., van den Broeke, J., Guleria, T., Bruni, C., Fantone, F., Touloupi, M., Iossifidis, D., Giménez Lorang, A., Sabbah, I., Farah, K., Baransi-Karkaby, K., Pidou, M., Reguer, A., Kleyböcker, A., Jährig, J., Vredenbregt, L., Thisgaard, P., D1.9 Start-up and intermediate results of plant operation from all case studies, 2023
- Sajjad Al-Amshawee, Mohd Yusri Bin Mohd Yunus, Abdul Aziz Mohd Azoddein, David Geraint Hassell, Ihsan Habib Dakhil, Hassimi Abu Hasan., Electrodialysis desalination for water and wastewater: A review, 2020





